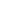
REACH Annex XVII Adds DMAC and NEP
On 3 June 2025, the European Commission published Regulation (EU) 2025/1090 in the Official Journal (OJ), amending Annex XVII to REACH (EC 1907/2006) regarding N,N-dimethylacetamide (DMAC) and 1-ethylpyrrolidin-2-one (NEP).
Chemical Toxicity
- N,N-dimethylacetamide (DMAC) and 1-ethylpyrrolidin-2-one (NEP) are dipolar aprotic solvents used in industrial settings and by professionals for formulating mixtures. Both substances are listed in Part 3 of Annex VI to Regulation (EC) No 1272/2008 as toxic.
- DMAC is listed as toxic to reproduction category 1B based on developmental toxicity and as acute toxic to category 4.
- NEP is listed as toxic to reproduction category 1B based on developmental toxicity.
New Entries to REACH Annex XVII
|
Entries |
Chemical |
CAS No. |
EC No. |
Requirements |
|
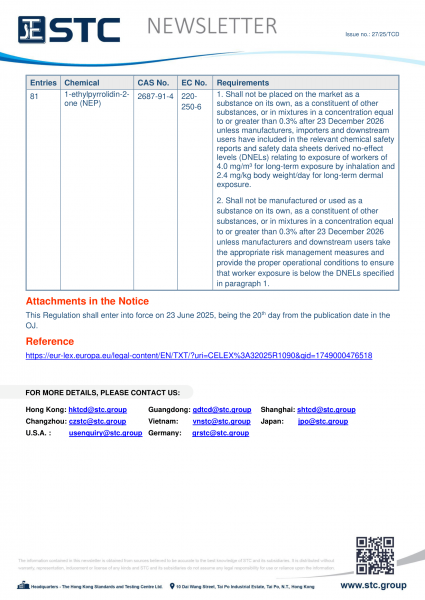
80 |
N,N-dimethylacetamide (DMAC) |
127-19-5 |
204-826-4 |
1. Shall not be placed on the market as a substance on its own, as a constituent of other substances, or in mixtures at a concentration equal to or greater than 0.3% after 23 December 2026 unless manufacturers, importers and downstream users have included in the relevant chemical safety reports and safety data sheets, derived no-effect levels (DNELs) relating to worker exposure of 13 mg/m3 for long-term inhalation and 1.8 mg/kg bw/day for long-term dermal exposure.
2. Shall not be manufactured or used as a substance on its own, as a constituent of other substances, or in mixtures in a concentration equal to or greater than 0.3% after 23 December 2026 unless manufacturers and downstream users take the appropriate risk management measures and provide the proper operational conditions to ensure that exposure of workers is below the DNELs specified in paragraph 1.
3. By way of derogation from paragraphs 1 and 2, the obligations laid down therein shall apply from 23 June 2029 concerning the placing on the market for use or use as a solvent in the production of man-made fibres. |
|
Entries |
Chemical |
CAS No. |
EC No. |
Requirements |
|
81 |
1-ethylpyrrolidin-2-one (NEP)
|
2687-91-4 |
220-250-6 |
1. Shall not be placed on the market as a substance on its own, as a constituent of other substances, or in mixtures in a concentration equal to or greater than 0.3% after 23 December 2026 unless manufacturers, importers and downstream users have included in the relevant chemical safety reports and safety data sheets derived no-effect levels (DNELs) relating to exposure of workers of 4.0 mg/m³ for long-term exposure by inhalation and 2.4 mg/kg body weight/day for long-term dermal exposure. 2. Shall not be manufactured or used as a substance on its own, as a constituent of other substances, or in mixtures in a concentration equal to or greater than 0.3% after 23 December 2026 unless manufacturers and downstream users take the appropriate risk management measures and provide the proper operational conditions to ensure that worker exposure is below the DNELs specified in paragraph 1. |
Attachments in the Notice
This Regulation shall enter into force on 23 June 2025, being the 20th day from the publication date in the OJ.
Reference
https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32025R1090&qid=1749000476518